Bradycardia in the Newborn
Impact factor 3,98
Published in a Q3 online journal
Abstract
Neonatal bradycardia in an awake baby of less than 90 beats per minute (2nd percentile) may occur as a reaction to a wide range of normal physiological as well as abnormal pathological conditions. Regarding diseases of the cardiac conduction system, congenital complete AV block (CCAVB) is the most important one (1). CCAVB can be present in a structurally healthy heart with autoimmune antibodies of the mother (isolated) or combined with a congenital heart defect (complex).
A significant bradycardia in newborns can be reflected either in hydrops caused by fetal heart failure or in signs of a low cardiac output or heart failure within a few hours to days after birth (2). A 1-day-old male newborn was admitted to our neonatal intensive care unit because of severe bradycardia and respiratory distress. He was born via caesarean terminated delivery at 36 weeks of gestation because of fetal bradyarrhythmia.
According to available data, the mother occasionally took antidepressants during pregnancy. At admission, he was alert but bradycardic (heart rate 50-76 beats per minute) in respiratory distress. Acid-base status: uncorrected metabolic acidosis with hypoxemia. Markers of inflammation and all bacterial cultures were negative. Electrocardiogram (ECG) showed sinus bradycardia with a heart rate around 75 beats per minute, with second degree AV block (Fig. 1).
Urgent echocardiogram showed patent foramen ovale. Given that there was no indication of an urgent implantation of a pacemaker, cardiac follow-up continued (24 Holter monitoring and echocardiogram). Respiratory instability was maintained during the first week of the stay in the intensive care unit as well as the pronounced bradycardia, while the third week resulted in clinical stabilization.
Normal sinus rhythm (144 beats per minute) was registered by the first 24h Holter monitoring, numerous supraventricular extrasystoles (SVES) - isolated, aberrantly conducted and non-conducted (Fig. 2). Throughout further follow-up, it was concluded that bradyarrhythmia occurred as a consequence of many non-conducted supraventricular extrasystoles. This conclusion was confirmed by the control 24h Holter monitoring six weeks later, during which sinus rhythm with rare and isolated SVES dominated, whereby the need for implantation of a pacemaker was unquestionably eliminated (Fig. 3).
Fetal bradyarrhythmia during the perinatal period can be the reason for an unjustifiable emergency delivery, without previous CCAVB evaluation or evaluation of some other, benign cause of slow heart rate. 24h Holter monitoring is crucial for accurate diagnosis and evaluation, as well as for the optimum time and method of treating heart rate disorder.
Introduction
Bradycardia in a newborn is defined as a heart rate below 2nd percentile relative to age, which corresponds to 91 beats per minute during the first week and 107 beats per minute during the first month of a baby's life (3, 4). There are no clinically significant differences between the sexes in the heart rate during the neonatal age; however, heart rate during rest and sleep is lower compared to that while awake, in particular while crying (5).
Table 1: Normal neonatal heart rate
| Age group | Heart rate (beats. min -1) |
|---|---|
| 0–1 days | 93–154 (123) |
| 1–3 days | 91–159 (123) |
| 3–7 days | 90–166 (129) |
| 7–30 days | 107–182 (149) |
From ref. [3,4].
2nd– 98th percentile (mean)
Bradyarrhythmia in children may occur as a reaction to a wide range of normal physiological as well as abnormal pathological conditions. Temporary bradycardia is present in 20-90% of healthy newborns, and most often it’s a consequence of a difficult delivery, because of a fetal hypoxia. Also, possible causes are hypothermia, hypoglycemia, acidosis, electrolyte disorders, raised tone of the vagus during feeding, sleeping, defaecation, increased intracranial pressure (6, 7).
CCAVB is the most important among diseases of the cardiac conduction system, which occurs in one per 14,000-20,000 live births (1). CCAVB can be combined with structurally healthy heart, caused by autoimmune antibodies of the mother (isolated) or with a congenital heart defect (complex), due to structural damages to the cardiac conduction system.
Temporary bradycardia in an asymptomatic healthy newborn spontaneously recedes during 48-72 hours; on the other hand, a severe and persistent bradycardia may reflect in hydrops, as a result of a fetal heart failure, or signs of a low cardiac output (irritability or lethargy, cold extremities, marbling of the skin or cyanosis) or heart failure (cardiomegaly, tachypnea) within a few hours to days after the birth (2).
Newborns, however, most often are asymptomatic. A typical ECG finding in a newborn with AV dissociation and bradycardia is regular, junctional rhythm with 60-80 beats per minute. The association between isolated neonatal AV block and maternal connective tissue disease is well established and ascribed to the presence of anti Ro/SSA and La-SSB antibodies in the mothers. Nearly every mother with an affected child has circulating antibodies. However, only 2 to 5% of women with known antibodies will have a first child with AV block (8).
Mortality rate in patients with neonatal AV block is still high, especially during the first 3 months of life (9). Acquired complete AV block is rare in neonates. It is mainly infective (viral myocarditis, HIV infection) or may be related to tumours (10). Neonates and infants with second or third degree AV block need a complete paediatric cardiologic work-up, including an echocardiogram (4).
Case Report
A 1-day-old male newborn was admitted to our neonatal intensive care unit because of severe bradycardia and respiratory distress. He was born via caesarean terminated delivery at 36 weeks of gestation because of fetal bradyarrhythmia, with birth weight of 2630g and Apgar score of 8/9.
According to anamnestic data it can be concluded that, during pregnancy, the mother occasionally took antidepressants. Family’s history was unremarkable. On the day of admission, the patient was first examined at general hospital, and because of persistent bradycardia he was referred to a tertiary neonatal center. At admission, he was alert but oedematous, in respiratory distress, with respiratory rate of 85 per minute and subcostal retractions. His pulse rate was 50-76 beats per minute accompanied by normal blood pressure (55/34/43 mmHg). Limb pulse oximetry was around 97%. Bradycardia without any cardiac murmur was found on cardiac examination.
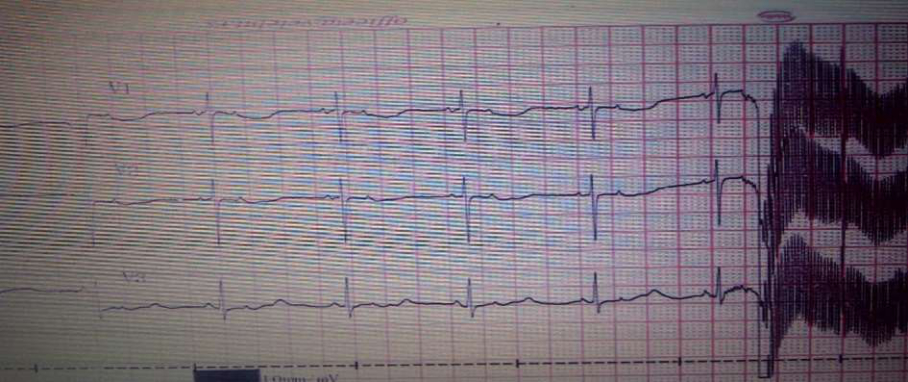
Fig.1 Electrocardiogram: Blocked atrial bigeminy which simulates sinus bradycardia and second degree AV block
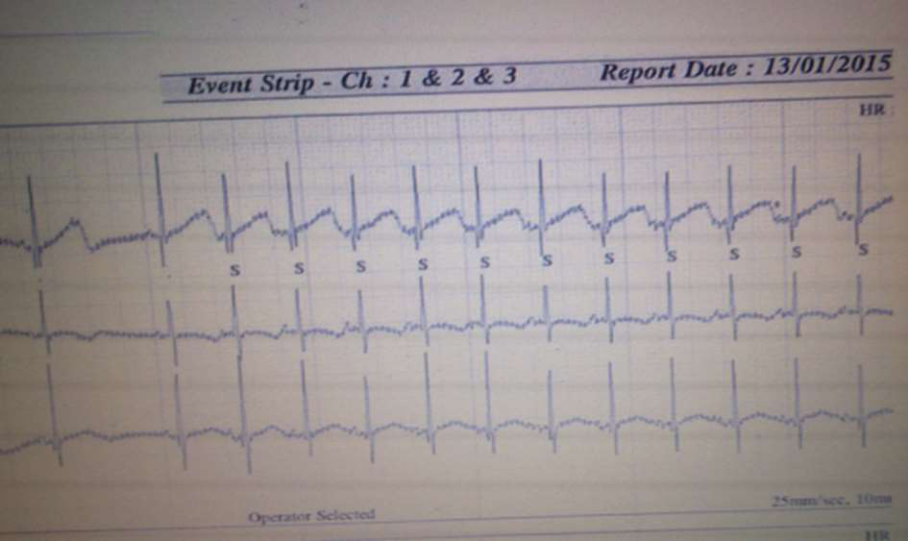
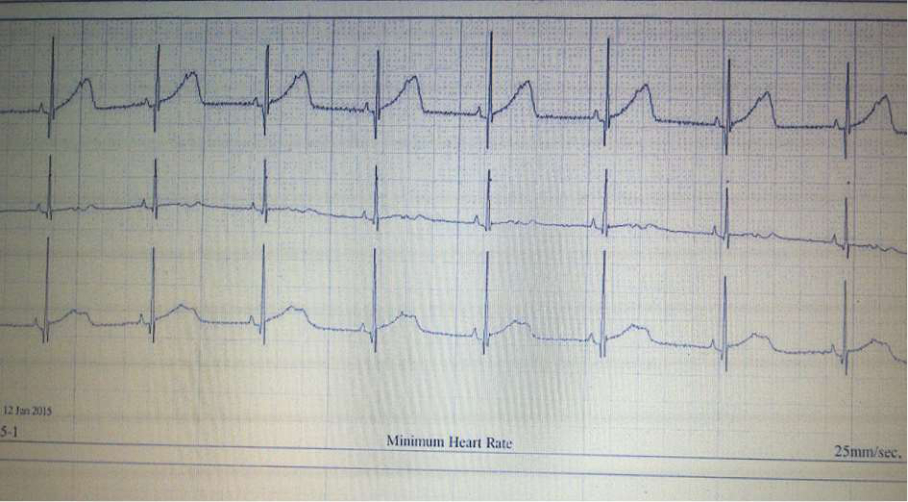
Fig.2 24h Holter monitoring: Blocked atrial bigeminy
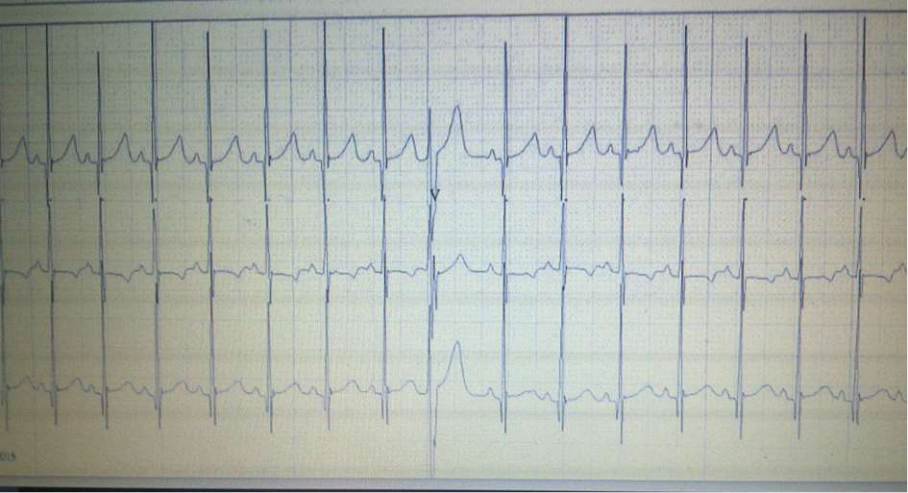
Fig.3 24h Holter monitoring: Sinus rhythm; Aberrant SVES
Other physical examination was without significant abnormalities. Initial laboratory data were as follows: hemoglobin 193 g/L, white blood count 16.000/mm3 with 11.26 (70%) neutrophil i 3.16 (19.6%) lymphocyte, platelat count 208.000/mm3. Acid-base status: uncorrected metabolic acidosis with hypoxemia (pH 7.25, BE -1.55 mmol/L, pCO2 8.51 kPa, pO2 5.61 kPa, HCO3 27.3 mmol/L, sO2 82.1 %). Other blood findings were without significant abnormalities. Markers of inflammation were negative: C-reactive protein 0.5 mg/L, procalcitonin 0.11 ng/ml. All bacterial cultures were negative. Report of viral analyses for TORCH diseases was negative.
Initial chest radiography revealed increased pulmonary vascular markings. Ultrasound of the central nervous system showed no significant abnormality. ECG showed sinus bradycardia with a heart rate around 75 beats per minute, with second degree AV block, and slightly prolonged QT interval (QTc 467 ms) ( Fig.1). Urgent echocardiogram showed structurally normal heart with patent foramen ovale.
24h Holter monitoring was conducted because of bradycardia maintenance, which registered normal sinus rhythm of 144 beats per minute and many isolated, aberrantly conducted and non-conducted SVES (Fig. 2). Respiratory instability was maintained during the first week of the stay in neonatal intensive care unit as well as the pronounced heart bradycardia, while the third week resulted in clinical stabilization.
Throughout further follow-up, it was concluded that bradyarrhythmia occurred as a consequence of many non-conducted supraventricular extrasystoles (SVES). This conclusion was confirmed by the control 24h Holter monitoring six weeks later, during which sinus rhythm with rare and isolated SVES dominated (Fig. 3), whereby the need for implantation of a pacemaker was unquestionably eliminated (Fig. 3).
Discussion
In our medical institution, every live-born child with bradycardia is first observed by neonatal transitional care team during the first two days after the birth, during which possible causes of bradycardia are investigated, ECG recording is conducted, testing results are waited for and, if necessary, various medical specialists are consulted.
If the ECG is abnormal, or any abnormality is found in the cardiovascular examination or if the baby becomes unwell, discussion with on-call paediatric cardiology team should be undertaken for urgent assessment including echocardiography. If the results obtained are normal and the heart rate has been normalized, the baby is discharged from the hospital. If bradycardia is maintained even after 48-72h, the newborn is admitted to the neonatal clinic, in order to implement urgent 24h Holter monitoring (Fig.4).
Bradycardia in this case was caused by non-conducted atrial bigeminy which resulted in false AV block 2:1. Relatively long periods of blocked atrial bigeminy may simulate sinus bradycardia. The distinction is important since blocked atrial bigeminy is most often benign while severe sinus bradycardia may accompany systemic illness (11).
Fetal bradyarrhythmia during the perinatal period can be the reason for an unjustifiable emergency delivery, without previous CCAVB evaluation or evaluation of some other, benign cause of a slow heart rate. 24h Holter monitoring is crucial for an accurate diagnosis and evaluation, as well as for the optimum time and method of treating heart rate disorder.
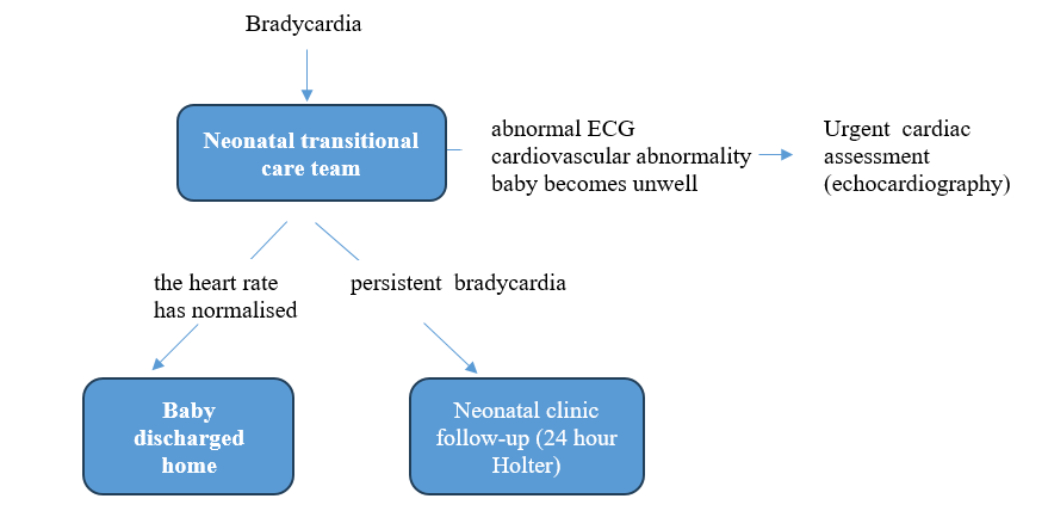
Fig 4. Neonatal bradycardia clinic follow-up algorithm
References
- Michealsson M et al. Congenital complete heart block: An international study of the natural history. Cardiovasc Clin. 1972;4:85–101.
- Veille JC, Covitz W. Fetal cardiovascular hemodynamics in the presence of complete atrioventricular block. Am J Obstet Gynecol. 1994;170:1258–62.
- Davignon et al. Normal ECG standards for infants and children. Pediatr Cardiol 1979; 1: 123–52.
- Schwartz PJ et al. Guidelines for the interpretation of the neonatal electrocardiogram, A Task Force of the European Society of Cardiology. Eur Heart J 2002; 23: 1329–44.
- Michelle S. et al. Neonatal Bradycardia. Progress in Pediatric Cardiology, Elsevier, 11 Ž2000. 19.24.
- Doniger SJ, Sharieff GQ. Pediatric dysrhythmias. Pediatr Clin North Am. 2006;53:85–105.
- Jaeggi E, Öhman A. Fetal and neonatal arrhythmias. Clin Perinatol. 2016;43:99–112.
- Brucato A, Frassi M, Franceschini F et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/ SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001; 44:1832–5.
- Buyon JP, Hiebert R, Copel J et al. Autoimmune-associated congenital heart block: long-term outcome of children and immunogenetic study. J Am Coll Cardiol 1998; 31: 1658–66.
- Wang, JN., Tsai, YC., Lee, WL. et al. Complete Atrioventricular Block Following Myocarditis in Children . Pediatr Cardiol 23, 518–521 (2002).
- Wren C. Cardiac arrhythmias in the fetus and newborn. Semin Fetal Neonatal Med. 2006;11:182–190.
© Drasko Nikcevic (2023). Bradycardia in the Newborn. MAR Pediatrics, 04 (09).
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
